co2 electron pair geometry and molecular geometry|Molecular geometry (VSEPR theory) (video) : Cebu One needs to know the Lewis structure in order to understand the molecular geometry of any given molecule. This structure helps in knowing the arrangement . Tingnan ang higit pa At STLBuildPro, we are well-versed in all aspects of new wall installation. Contact us for all your drywall and home remodeling needs. Call 314-669-9741.
PH0 · Predicting Electron
PH1 · Molecular geometry (VSEPR theory) (video)
PH2 · Molecular Geometry – Introductory Chemistry
PH3 · CO2 Lewis Structure, Molecular Geometry, Molar Mass
PH4 · CO2 Lewis Structure, Molecular Geometry and Hybridization
PH5 · CO2 Lewis Structure, Molecular Geometry and
PH6 · CO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagr
PH7 · CO2 Lewis Structure, Hybridization, Molecular
PH8 · CO2 Lewis Structure,
PH9 · 8.6: Molecular Geometries
PH10 · 7.2 Electron Pair Geometry versus Molecular Structure
PH11 · 5.9: Molecular Geometry
Gaming law is the set of rules and regulations that apply to the gaming or gambling industry. Gaming law is not a branch of law in the traditional sense but rather is a collection of several areas of law that include criminal law, regulatory law, constitutional law, administrative law, company law, contract law, and in some jurisdictions, competition law.
co2 electron pair geometry and molecular geometry*******The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Here in . Tingnan ang higit paOne needs to know the Lewis structure in order to understand the molecular geometry of any given molecule. This structure helps in knowing the arrangement . Tingnan ang higit pa
The electronic configuration of the Carbon atom in its ground state is 1s22s22p2, and that of an Oxygen atom is 1s22s2p4. When the electrons are in an excited state, they jump . Tingnan ang higit pa
So molecular geometry is those which include only the atom while determining the shape of the molecule. Whereas electron geometry includes all electron pairs. .
Predicting Electron Pair Geometry and Molecular Structure. The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular structures: Write the Lewis . CO2 Molecular Geometry & Shape. In a CO2 molecule, the carbon atom is in the center double bonded with two oxygen atoms .
Lewis Structure of Carbon Dioxide. Carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon. The carbon-oxygen ratio in a CO 2 molecule is 1:2. Two double bonds .In calculating electronic geometry we use the Valence Shell Electron Pair Repulsion (VSEPR) model, which states that the lowest geometry for electronic orbitals around a positive nucleus is for the orbitals to be as .
The C in CO 2 has a linear electron-pair geometry and a linear molecular structure/shape. Both of these are the same since there are no lone pairs on the C .Identify the electron-pair geometry based on the number of regions of electron density: linear, trigonal planar, tetrahedral, trigonal bipyramidal, or octahedral ( Figure 7.2.6, first column). Use the number of lone pairs .
co2 electron pair geometry and molecular geometry Molecular geometry (VSEPR theory) (video) About. Transcript. Valence Shell Electron Pair Repulsion (VSEPR) theory is used to predict the three-dimensional shapes of molecules based on the repulsion between electron pairs around a central atom. Learn how to use Lewis structures to count electron .
The main geometries without lone pair electrons are: linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral. Key Terms. VSEPR Theory: a chemistry model used to predict the shape of individual .
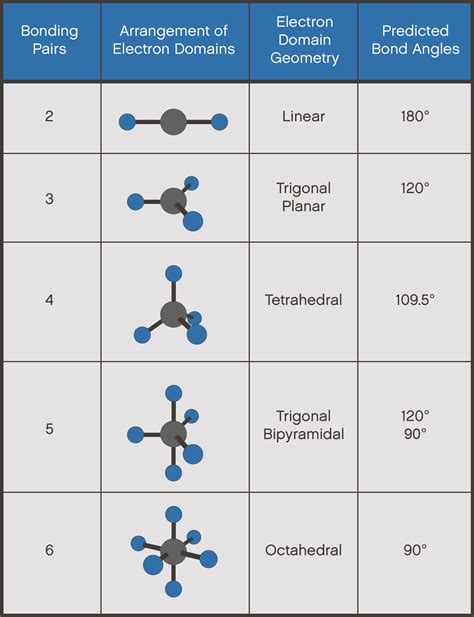
In the CO2 lewis structure, there is a total of 4 lone pairs present. Two lone pairs on each oxygen atom. The electron geometry of CO2 is also linear. The bond angle of CO2 is 180º. Since it is linear in .co2 electron pair geometry and molecular geometry In the CO2 lewis structure, there is a total of 4 lone pairs present. Two lone pairs on each oxygen atom. The electron geometry of CO2 is also linear. The bond angle of CO2 is 180º. Since it is linear in .Figure 10.2.2 ): (CC BY-NC-SA; anonymous) The two oxygens are double bonded to the sulfur. The oxygens have 2 lone pairs while sulfur had one lone pair. 3. There are two bonding pairs and one lone pair, so the structure is designated as AX 2 E. This designation has a total of three electron pairs, two X and one E.Molecular geometry (VSEPR theory) (video) In this video we look at the electron geometry for CO2 (Carbon Dioxide). Because the Carbon dioxide molecule has two electron domains (two oxygen atoms and n.Since water has two lone pairs it's molecular shape is bent. According to the VSEPR theory, the electrons want to minimize repulsion, so as a result, the lone pairs are adjacent from each other. CO 2: Carbon dioxide has two electron groups and no lone pairs. Carbon dioxide is therefore linear in electron-group geometry and in molecular .Examples: In a water molecule, H 2 O two of the central oxygen atom’s valence electrons form two bond pairs with the hydrogen atoms, while the remaining four electrons form two lone pairs. Therefore, the molecular geometry of water is bent and the electron geometry of water is tetrahedral. Ammonia, NH 3, is another example with different molecular and .Thus, the electron-pair geometry is tetrahedral and the molecular structure is bent with an angle slightly less than 109.5°. In fact, the bond angle is 104.5°. Figure 7.2.7. (a) H2O H 2 O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry.

Linear Electron Pair Geometry and Molecular Shape. Carbon dioxide has a linear electron pair geometry and a linear molecular geometry. The bonds are 180 o apart.. A linear molecular shape is represented by AX 2 where the letter A is the central atom, X corresponds to the bonds and E corresponds to the lone pairs of electrons that .
Carbonate ion (CO32-) Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. CO 32- is the chemical formula for carbonate ion, a polyatomic ion composed of 1 carbon and 3 oxygen atoms. It is present in a carbonate salt i.e., a salt of carbonic acid (H 2 CO 3 ).
You forgot your password? You can easily request a new password here.
co2 electron pair geometry and molecular geometry|Molecular geometry (VSEPR theory) (video)